Abstract
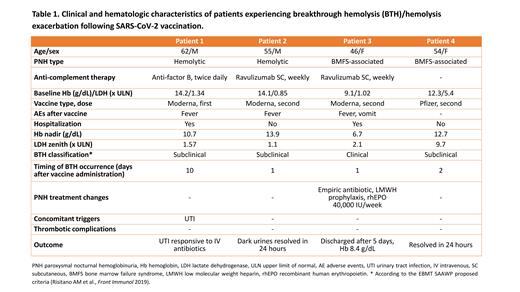
SARS-CoV-2 infection and vaccination have raised concerns in paroxysmal nocturnal hemoglobinuria (PNH). In fact, PNH patients carry an increased infectious risk secondary to complement inhibition treatment or associated bone marrow failure (BMF), and may therefore benefit from preventive strategies such as vaccinations. On the contrary, vaccines can be numbered among inflammatory complement amplifiers (e.g., infections, traumas, surgery), potentially triggering a disease exacerbation. In PNH patients on complement inhibitors, this phenomenon has been defined pharmacodynamic breakthrough hemolysis (BTH). Based on isolated reports of BTH following SARS-CoV-2 vaccines, we conducted a survey among 5 Italian reference centers to evaluate complications and BTH occurrence in PNH patients who completed the SARS-CoV-2 vaccination schedule from January, 2 2021 until the time of writing. Adverse events, hematologic and hemolytic parameters were recorded within 7-10 days before and after each dose of vaccine. A total of 67 patients (females/males 43/24, median age 47.6 years, range 21-90.5) were eligible for the analysis. According to the International PNH Interest Group classification, 45 patients suffered from hemolytic PNH, 20 from PNH in the context of BMF syndromes (aplastic anemia or myelodysplastic syndrome), and 2 from subclinical PNH. Fifty-five subjects (82%) were on regular complement inhibition therapy, i.e., eculizumab (N=35), ravulizumab (N=13), subcutaneous anti-C5 (N=3), anti-factor B (N=2) and ravulizumab + anti-factor D combination (N=2). Vaccines (Comirnaty/Pfizer-BioNTech N=53, mRNA-1273/Moderna N=12, and ChAdOx1 nCov-19/AstraZeneca N=2) were complessively well-tolerated, with 3 non-hematologic adverse events after the first dose (2 fever and 1 exercise-induced tachycardia, grade 1 according to CTCAE v5.0) and 2 after the second one (fever, accompanied by vomit in one patient, grade 1). During the observation period, 3 BTH and 1 hemolytic exacerbation were recorded (5.9% of patients), as detailed in Table 1. The most severe episode occurred in a young woman (Patient 3) on subcutaneous ravulizumab who experienced a hemoglobin (Hb) drop >2 g/dL, marked clinical signs of intravascular hemolysis and lactate dehydrogenase (LDH) increase >1.5 x upper limit of normal (ULN) from baseline, which is considered a clinical BTH according to the criteria proposed by the Severe Aplastic Anemia Working Party of the European group for Bone Marrow Transplantation. The patient required hospitalization for additional treatment with recombinant erythropoietin and anti-thombotic/bacterial prophylaxis. The second more severe BTH was registered in a male patient (Patient 1) on oral anti-factor B who experienced a Hb drop >2 g/dL without an overt hemolytic flare, and required hospitalization for intravenous antibiotic therapy (concomitant urinary tract infection). The remaining two patients experienced a subclinical BTH (Patient 2) and a hemolytic flare (Patient 4, not on complement inhibition). On the whole, a median delta variation from usual values of Hb and LDH of -25% (range -26+3%) and +80% (+18+105%) were observed, respectively. Of note, 3 episodes occurred after the second dose of vaccine, generally within 24-48 hours. Anti-complement drugs were not modified/discontinued in any of the 3 patients on regular treatment. Patients not experiencing BTH (94.1%) showed stable hematologic parameters after the first dose (Hb/LDH median delta variations from baseline -1%/+1%, range -14+12%/-32+40%) and the second dose of vaccine (Hb/LDH median delta variations from baseline +1%/0%, range -18+47%/-76+41%). Of note, 4 patients with a previous SARS-CoV-2 infection completed the vaccination without any complication/PNH exacerbation.
In conclusion, this survey shows that BTH/hemolytic flares following SARS-CoV-2 vaccines are observed in about 6% of PNH patients, may be clinically relevant but manageable, and should not discourage vaccination. BTH has been registered mostly few days after the second dose of vaccine, suggesting a "booster" effect favoring a higher inflammatory response. Watchful clinical and laboratory monitoring is advised, in order to promptly recognize severe hemolytic flares in both treated and naïve patients.
Fattizzo: Novartis: Speakers Bureau; Kira: Speakers Bureau; Alexion: Speakers Bureau; Annexon: Consultancy; Momenta: Honoraria, Speakers Bureau; Apellis: Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Bianchi: Agios pharmaceutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sica: Jazz Pharma: Consultancy; Alexion: Consultancy. Barcellini: Novartis: Other: Invited speaker, Research Funding; Agios: Membership on an entity's Board of Directors or advisory committees; Alexion Pharmaceuticals, Inc: Membership on an entity's Board of Directors or advisory committees, Other: Invited speaker, Research Funding; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal